Welcome to the Properties of Water Crossword Puzzle Answer Key, your comprehensive guide to the fundamental characteristics of this life-sustaining substance. This key unlocks a world of scientific knowledge, providing answers to the most intriguing questions about water’s physical, chemical, biological, and environmental properties.
From its unique density to its remarkable ability to dissolve a myriad of substances, water’s properties play a pivotal role in shaping our planet and sustaining life as we know it. Delve into this fascinating journey to unravel the secrets of H2O and gain a deeper understanding of its profound impact on our world.
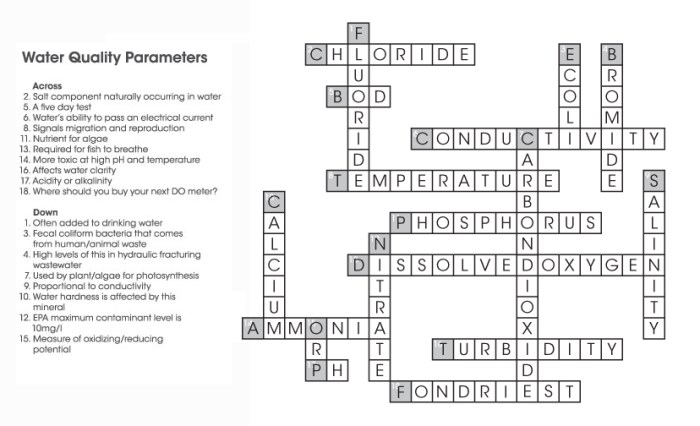
Properties of Water
Water, the elixir of life, possesses remarkable properties that underpin its vital role in sustaining life and shaping our planet.
Physical Properties of Water
Water exhibits unique physical properties that contribute to its exceptional behavior.
Density and Aquatic Life
Water’s density is highest at 4°C. This anomaly allows ice to float, creating an insulating layer that protects aquatic life in cold environments.
Specific Heat Capacity
Water has a high specific heat capacity, meaning it can absorb or release large amounts of heat without significant temperature changes. This property plays a crucial role in regulating Earth’s temperature.
Surface Tension
Water’s high surface tension enables it to form droplets and resist penetration. This property facilitates capillary action, allowing water to rise in narrow tubes.
Chemical Properties of Water
Water’s chemical properties make it a versatile solvent and a key participant in numerous reactions.
Polarity
Water is a polar molecule, meaning it has a partial positive charge at one end and a partial negative charge at the other. This polarity allows it to dissolve a wide range of substances.
Hydrolysis Reactions
Water acts as a reactant in hydrolysis reactions, breaking down larger molecules into smaller components.
pH
Water’s pH is a measure of its acidity or alkalinity. It plays a vital role in biological systems, influencing enzyme activity and cellular processes.
Biological Significance of Water
Water is essential for all known life forms.
Cellular Processes
Water comprises over 70% of living cells and is involved in metabolism, transportation, and waste removal.
Photosynthesis and Respiration
Water is a reactant in photosynthesis and a byproduct of cellular respiration, two fundamental processes for life on Earth.
Body Temperature Regulation
Water’s high specific heat capacity and evaporation properties aid in maintaining body temperature in living organisms.
Environmental Implications of Water
Water is a critical component of the Earth’s ecosystem.
Water Cycle
Water undergoes a continuous cycle of evaporation, condensation, and precipitation, shaping the Earth’s climate.
Water Pollution, Properties of water crossword puzzle answer key
Water pollution threatens aquatic ecosystems and human health. It can disrupt food chains, contaminate drinking water, and harm aquatic life.
Water Conservation
Water conservation strategies, such as reducing water consumption and recycling wastewater, are essential for sustainable development.
Examples of Water’s Properties in Everyday Life
Water’s unique properties have myriad applications in daily life.
| Property | Application |
|---|---|
| Density | Floating boats, creating ice cubes |
| Specific Heat Capacity | Cooling systems, heating pads |
| Surface Tension | Water droplets, soap bubbles |
| Polarity | Dissolving sugar, cleaning with soap |
| pH | Maintaining aquarium water, treating acidic soil |
FAQ Compilation: Properties Of Water Crossword Puzzle Answer Key
What is the significance of water’s high specific heat capacity?
Water’s high specific heat capacity allows it to absorb and release large amounts of heat without significant temperature changes, making it an excellent temperature regulator in both natural and engineered systems.
How does water’s polarity contribute to its ability to dissolve substances?
Water’s polarity, resulting from the uneven distribution of electrons, enables it to form hydrogen bonds with other polar molecules and ions, facilitating their dissolution.
What is the role of water in cellular processes?
Water is a crucial component of cells, constituting around 70% of their volume. It participates in numerous cellular processes, including metabolism, transportation, and waste removal.