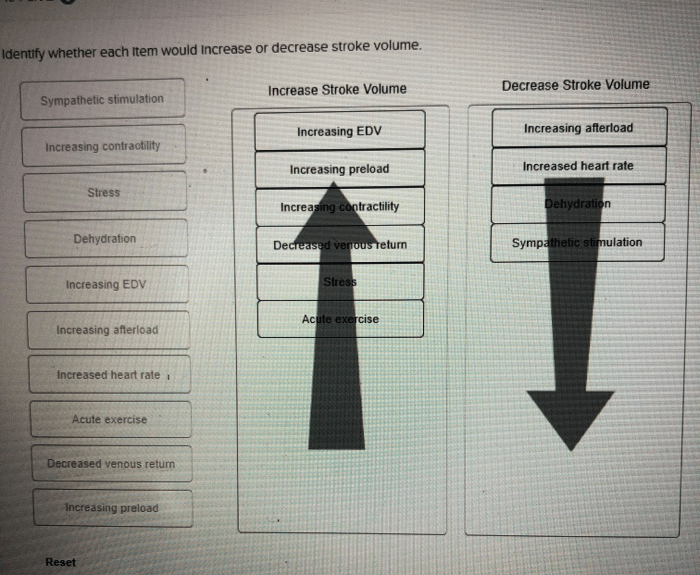
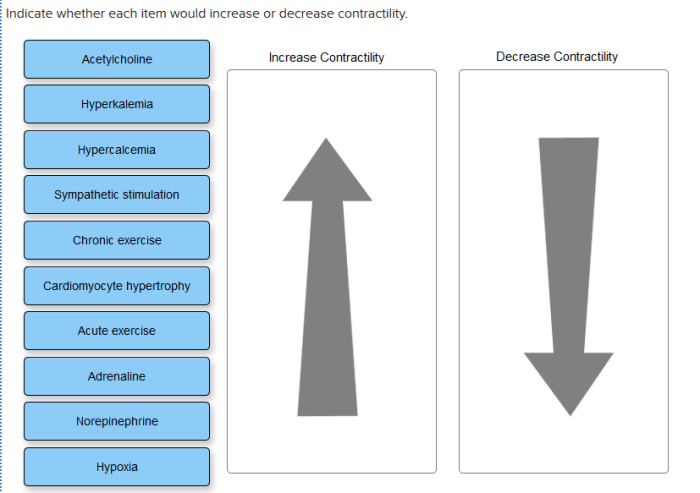
Indicate whether each item would increase or decrease contractility. – Contractility, the ability of muscles to shorten and generate force, is a fundamental physiological process. Understanding the factors that influence contractility is crucial for comprehending muscle function and movement. This article delves into the intricate mechanisms that govern contractility, exploring the roles of calcium, myosin phosphorylation, sarcomere length, muscle fiber type, neural input, and the effects of drugs and toxins.
From the interplay of calcium ions to the structural dynamics of myosin, this exploration unveils the complex symphony that orchestrates muscle contraction. By unraveling these factors, we gain deeper insights into the physiological underpinnings of movement, paving the way for advancements in fields such as exercise science, rehabilitation, and pharmacology.
Effect of Calcium on Contractility
Calcium ions play a crucial role in muscle contraction by triggering the interaction between actin and myosin filaments. When calcium levels in the muscle cell increase, it binds to troponin, a protein complex associated with the thin actin filaments. This binding causes a conformational change in troponin, exposing the myosin-binding sites on actin, allowing myosin heads to attach and initiate muscle contraction.
Impact of Increased Calcium Levels on Contractility, Indicate whether each item would increase or decrease contractility.
Increased calcium levels enhance contractility by facilitating the formation of more cross-bridges between actin and myosin filaments. This results in a greater number of myosin heads interacting with actin, generating more force and shortening the muscle fiber. The increase in calcium concentration also promotes the release of calcium from the sarcoplasmic reticulum, a specialized organelle that stores calcium ions within muscle cells, further enhancing contractility.
Impact of Myosin Phosphorylation on Contractility
Myosin is a motor protein responsible for generating force during muscle contraction. Myosin phosphorylation, the addition of phosphate groups to myosin, affects its interaction with actin and influences contractile force.
Role of Myosin Phosphorylation in Contractile Force
Myosin phosphorylation enhances its affinity for actin, promoting stronger binding between the two proteins. This increased binding allows for more efficient force generation and contributes to increased contractility. Phosphorylation also affects the kinetics of the myosin-actin interaction, influencing the rate of cross-bridge formation and detachment, which can further modulate contractile force.
Role of Sarcomere Length on Contractility: Indicate Whether Each Item Would Increase Or Decrease Contractility.
Sarcomere length, the distance between two adjacent Z-lines in a muscle fiber, plays a critical role in determining contractility.
Optimal Sarcomere Length for Maximum Contractility
Optimal sarcomere length maximizes contractility because it allows for optimal overlap between actin and myosin filaments. When sarcomere length is too short or too long, the overlap between filaments is reduced, limiting the number of cross-bridges that can form and decreasing contractile force.
Common Queries
What is the primary role of calcium in muscle contraction?
Calcium ions trigger muscle fiber shortening by binding to troponin, initiating a conformational change that exposes myosin-binding sites on actin.
How does myosin phosphorylation affect contractile force?
Myosin phosphorylation increases its affinity for actin, enhancing the formation of cross-bridges and ultimately increasing contractile force.
What is the optimal sarcomere length for maximal contractility?
Optimal sarcomere length is typically around 2.2 micrometers, where the overlap between actin and myosin filaments is maximized.