Classify the pair of compounds as constitutional isomers or stereoisomers – In chemistry, isomerism is a fundamental concept that describes the existence of compounds with the same molecular formula but distinct structural arrangements. Classifying compounds as constitutional isomers or stereoisomers is crucial for understanding their chemical properties and reactivity. This article provides a comprehensive overview of constitutional and stereoisomers, their key characteristics, and the steps involved in their classification.
1. Constitutional Isomers
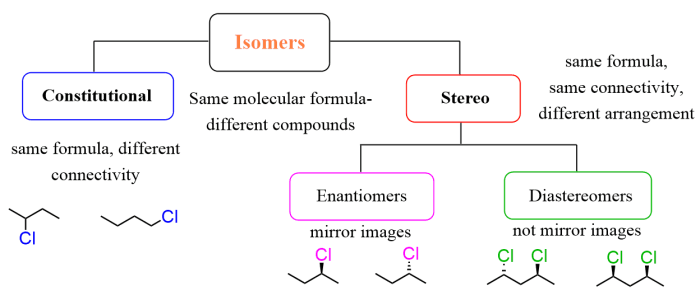
Constitutional isomers, also known as structural isomers, are compounds that have the same molecular formula but different structural formulas. The difference in their structures results in different chemical and physical properties.
Key characteristics that differentiate constitutional isomers from other types of isomers include:
- Different connectivity of atoms
- Different arrangement of functional groups
- Different molecular frameworks
Examples of constitutional isomers with different molecular formulas:
- Butane (C 4H 10)
- Isobutane (C 4H 10)
- Propene (C 3H 6)
- Cyclopropane (C 3H 6)
| Constitutional Isomer | Molecular Formula | Structural Formula | Physical State | Boiling Point (°C) |
|---|---|---|---|---|
| Butane | C4H10 | CH3CH2CH2CH3 | Gas | -0.5 |
| Isobutane | C4H10 | (CH3)3CH | Gas | -11.7 |
| Propene | C3H6 | CH3CH=CH2 | Gas | -47.7 |
| Cyclopropane | C3H6 | C3H6 | Gas | -33 |
2. Stereoisomers
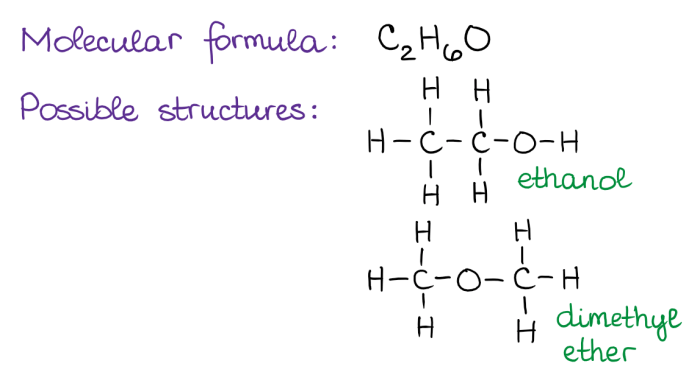
Stereoisomers are compounds that have the same molecular formula and connectivity of atoms but differ in the spatial arrangement of their atoms. This difference in spatial arrangement can lead to different chemical and physical properties.
The different types of stereoisomers include:
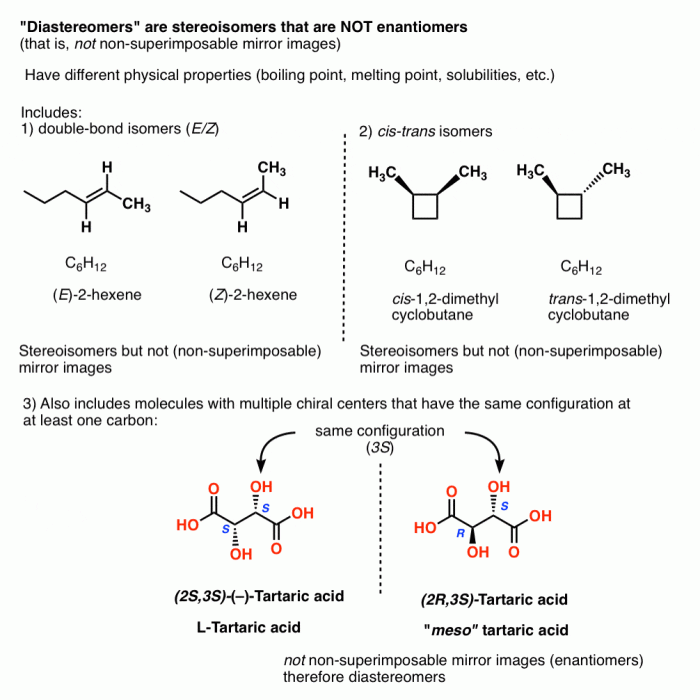
- Enantiomers: Stereoisomers that are non-superimposable mirror images of each other.
- Diastereomers: Stereoisomers that are not mirror images of each other and are not superimposable.
Illustrations demonstrating the spatial arrangement of atoms in stereoisomers:
- Enantiomers:
- Diastereomers:
- Key differences between enantiomers and diastereomers:
- Enantiomers have identical physical properties except for their interaction with chiral molecules.
- Diastereomers have different physical properties.
- Enantiomers rotate plane-polarized light in opposite directions.
- Diastereomers rotate plane-polarized light in different directions but not in opposite directions.
3. Classifying Compounds as Constitutional or Stereoisomers
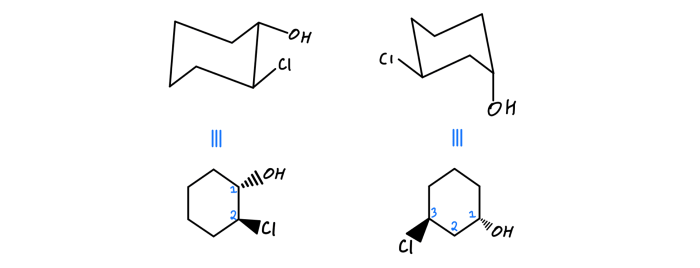
To classify compounds as constitutional or stereoisomers, follow these steps:
- Determine the molecular formula of the compound.
- Draw the structural formula of the compound.
- Identify the different ways the atoms can be connected to each other.
- If the compounds have different molecular formulas, they are constitutional isomers.
- If the compounds have the same molecular formula but different structural formulas, they are stereoisomers.
The importance of molecular structure in determining the type of isomerism:
- Constitutional isomers have different molecular structures.
- Stereoisomers have the same molecular structure but different spatial arrangements of their atoms.
Flowchart to illustrate the decision-making process for classifying compounds as constitutional or stereoisomers:
4. Applications of Isomerism
Isomerism has various practical applications in different fields:
- Chemistry:
- Understanding reaction mechanisms and selectivity
- Designing and synthesizing new compounds with specific properties
- Medicine:
- Developing drugs with specific biological activity
- Understanding drug metabolism and interactions
- Materials science:
- Designing polymers with specific properties
- Understanding the structure and properties of materials
Examples of how isomerism has influenced scientific advancements:
- The discovery of the different isomers of glucose led to a better understanding of carbohydrate metabolism.
- The development of enantioselective catalysts has enabled the synthesis of chiral compounds with high enantiomeric purity.
- The understanding of isomerism in polymers has led to the development of new materials with tailored properties.
Expert Answers: Classify The Pair Of Compounds As Constitutional Isomers Or Stereoisomers
What is the key difference between constitutional isomers and stereoisomers?
Constitutional isomers have different molecular formulas and connectivity, while stereoisomers have the same molecular formula but differ in the spatial arrangement of their atoms.
How can you determine if a pair of compounds are constitutional isomers or stereoisomers?
By examining their molecular formulas and structures, you can identify whether they are constitutional isomers (different molecular formulas) or stereoisomers (same molecular formula but different spatial arrangement).




